text

فيديو
Untraced, but not forgotten- We owe our children with drug-resistant TB - INT








تعرف على مهمتنا وميثاقنا ومبادئنا.
اقرأ المزيد حول أطباء بلا حدودتعرّف على آلية عمل منظمة أطباء بلا حدود وقدرتنا على الاستجابة بسرعة بفضل الإمدادات والخدمات اللوجيستية.
اقرأ المزيد كيف نعملاطلع على نظامنا الإداري وجمعياتنا. اعثر على دليل مرئي سريع لمكاتبنا حول العالم.
اقرأ المزيد إدارة منظمتنااطلع على تقاريرنا المالية والتقارير السنوية للأنشطة، واكتشف مصدر أموالنا وكيفية إنفاقها.
اقرأ المزيد التقارير المالية وتقارير الأنشطةاطلع على السياسات والتقارير والخطط المتعلقة بكيفية تعاملنا مع قضايا مثل العنصرية وتقليل بصمتنا الكربونية وضمان توافق سلوكاتنا مع أعلى المعايير الأخلاقية.
اقرأ المزيد المساءلةتواصل مع مكاتبنا حول العالم.
اقرأ المزيد اتصل بناتقدّم أطباء بلا حدود المساعدات الطبيّة إلى الأشخاص المتضررين من النزاعات والأوبئة والكوارث أو المحرومين من الرعاية الصحية.
كل الملفات والقضايا الإنسانية الأنشطة حسب البلد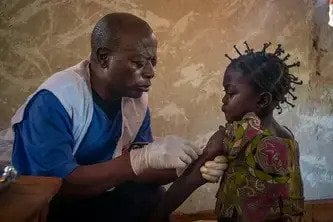
تقدم فرقنا الرعاية لمرضى حول العالم يعانون من أمراض وحالات صحية مختلفة. اقرأوا حول الاحتياجات الطبية الرئيسية الني نستجيب لها في الميدان.
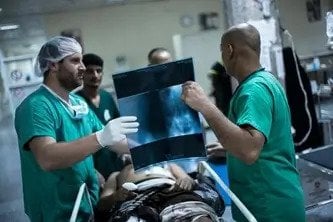
تعمل فرقنا في ظلّ أزمات إنسانية متنوعة حول العالم. اقرأوا حول استجاباتنا لأبرز الأزمات والتحديات التي تواجه مرضانا في الحصول على الرعاية.

اقرأوا مقالات مفصّلة تلخّص عملنا خلال أزمات أو أحداث كانت أو لا تزال ذات وقع كبير على مرضانا.
تقدم منظمة أطباء بلا حدود، في أكثر من 70 بلدا، المساعدة الإنسانية الطبية لإنقاذ الأرواح وتخفيف معاناة الناس في حالات الأزمات
عرض كل البلدانبرامج ومشاريع الحملة
قم بزيارة الموقع حملة أطباء بلا حدود لتوفير الأدوية الأساسيّةآحر التقارير والاصدارات حول العمل الانساني
قم بزيارة الموقع مركز أطباء بلا حدود للفكر والمعرفة في مجال العمل الانسانيآخر الأبحاث والاصدارات حول أحسن السبل لايصال المساعدات الانسانيّة
قم بزيارة الموقع مركز البحوث حول الممارسات والتحديات في مجال العمل الانسانيأفكار ونقاشات حول تحديات العمل الانساني
قم بزيارة الموقع مركز أطباء بلا حدود للتفكير حول المشاريع الانسانيّةتحليل و حوارات و مناظرات حول القضايا الانسانيّة
قم بزيارة الموقع مركز أطباء بلا حدود للتحليلمركز التخزين والتعبئة والامدادات و
قم بزيارة الموقع مركز أطباء بلا حدود للامدادات واللوجستياتمركز تخزين المعدات الطبيّة قبل ارسالها الى الميدان
قم بزيارة الموقع مركز أطباء بلا اللوجستياتقسم للتأكد من نوعيّة الشراءات و مطابقتها للجودة
قم بزيارة الموقع قسم الشراءات ببروكسالوحدة مختصة في الدعم التقني لعلاج الامراض مثل الشاغاس
قم بزيارة الموقع الوحدة الطبيّة في البرازيلالمراجع والبروتوكولات الطبيّة المستخدمة من قبل المنظمة
قم بزيارة الموقع المراجع الطبيّة لأطباء بلا حدودأخر التحاليل و البحوث في مجال علم الاوبئة
قم بزيارة الموقع مركو البحث في علم الأوبئةوحدة تقييم المشاريع والتدخلات الانسانيّة
قم بزيارة الموقع وحدت التقييمMSF works with LGBTQI+ populations in many settings over the last 25-30 years. LGBTQI+ people face healthcare disparities with limited access to care and higher disease rates than the general population.
GO TO SITE LGBTQI+ Inclusion in Health Settingsأبحاث ودراسات حول التدخلات الانسانيّة و نجاعتها
قم بزيارة الموقع وحدة البحث حول العمليّات الإنسانيّة في لوكسمبورغThe Intersectional Benchmarking Unit collects and analyses data about local labour markets in all locations where MSF employs people.
GO TO SITE Intersectional Benchmarking UnitTo upskill and provide training to locally-hired MSF staff in several countries, MSF has created the MSF Academy for Healthcare.
GO TO SITE MSF Academy for Healthcareموقع يشرح القانون الدولي الانساني
قم بزيارة الموقع القانون الدولي الانسانيThe MSF Paediatric Days is an event for paediatric field staff, policy makers and academia to exchange ideas, align efforts, inspire and share frontline research to advance urgent paediatric issues of direct concern for the humanitarian field.
GO TO SITE MSF Paediatric Daysمؤسسة للحوار وتبادل الخبرات حول الامدادات و العمل الطبي الانساني
قم بزيارة الموقع مؤسسة أطباء بلا حدودمبادرة للبحث وتطوير أدوية جديدة للأمراض المهملة
قم بزيارة الموقع مبادرة توفير أدوية الأمراض المهملةOur digital portal dedicated to sharing the latest medical evidence from our humanitarian activities around the globe.
GO TO SITE MSF Science PortalNoma is a preventable and treatable neglected disease, but 90 per cent of people will die within the first two weeks of infection if they do not receive treatment.
GO TO SITE NomaThe TIC is aiming to change how MSF works to better meet the evolving needs of our patients.
GO TO SITE TICMSF's telemedicine hub aims to overcome geographic barriers for equitable, accessible, and quality patient care.
GO TO SITE TelemedicineLaunched in 2012, the MSF Sweden Innovation Unit deploys a human-centered approach for promoting a culture of innovation within MSF.
GO TO SITE Sweden Innovation Unittext








