- Lifesaving TB medicine is still unaffordable and out of reach for children in high-burden countries
- The World Health Organization (WHO) recommends children of all ages have access to all-oral treatment for drug-resistant tuberculosis.
- MSF calls on governments of high-burden countries to allow production of these lifesaving drugs to be affordable for all.
Geneva – The World Health Organization (WHO) recently released new rapid guidance recommending that children of all ages with drug-resistant tuberculosis (DR-TB) have access to all-oral treatment using the drugs bedaquiline and/or delamanid.
However, adopting these new recommendations in high TB burden countries requires access to the paediatric formulations of bedaquiline (produced by Johnson & Johnson) and delamanid (produced by Otsuka and its local partner Viatris).
Médecins Sans Frontières (MSF) therefore calls on governments of high-burden countries to take measures to overcome patent barriers and allow production of these lifesaving drugs through generic manufacturers.
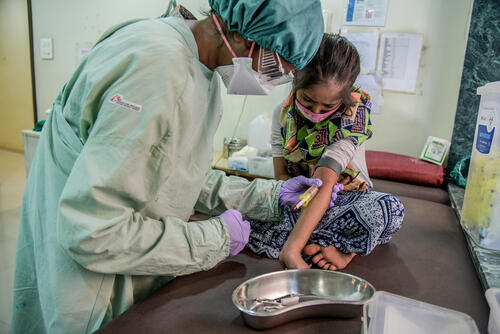
“The WHO’s updated rapid guidance is an important step forward for younger children with drug-resistant TB to receive all-oral treatment without painful injectable drugs,” says Dr. Mabel Morales, MSF medical coordinator in India.
“However, this new guidance will remain a distant reality for children unless access barriers to paediatric formulations of bedaquiline and delamanid are overcome, allowing them to be rolled out by national TB programmes in all high-burden countries,” says Morales.
In addition to the slow pace of national guideline changes, access to children’s formulations has been a challenge in high TB burden countries due to high prices and the lack of registration and generic competition.
This new guidance will remain a distant reality for children unless access barriers to paediatric formulations of bedaquiline and delamanid are overcome.Dr. Mabel Morales, MSF medical coordinator in India
In our teams’ experience, the registration and supply of paediatric formulations are not prioritised by pharmaceutical corporations, and having only one manufacturer for a given drug often results in these formulations being more expensive than the adult versions.
“The high price of delamanid of $1,700 per treatment course has significantly limited access in many countries,” says Morales. “In India, negotiations with Otsuka and Viatris have been unsuccessful, with the manufacturers refusing to lower the price to the $942 – currently being offered to South Africa by Viatris.
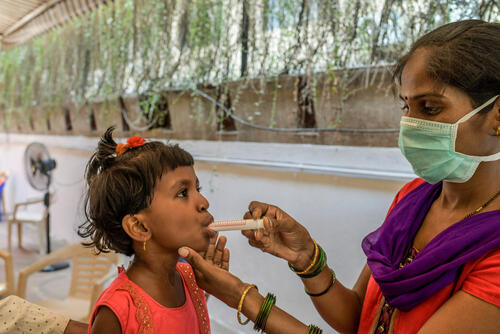
“The price of paediatric formulations of bedaquiline also remain too high. It’s time to smash the status quo: pharmaceutical corporations Johnson & Johnson and Otsuka must open up to generic supply and lower prices in order for TB programmes to scale up all-oral treatment regimens,” Morales says.
“As treatment providers, we see kids with drug-resistant TB on an almost daily basis in our independent clinic in Mumbai,” says Morales. “We no longer want to see these younger children suffer the terrible side effects of the older and painful injection-based drugs, when safer and more effective oral medicines are available elsewhere.”
Paediatric formulations of delamanid: dispersible 25mg tablets are only available under Otsuka’s compassionate use programme for children weighing more than 10kg, until the end of 2021. The drug will be supplied next year through the Global Drug Facility (GDF) at a currently unknown price. For children and adolescents weighing more than 30kg, GDF is supplying Otsuka’s adult 50mg tablets at US$1700 for a six-month treatment course. Patent barriers prevent generic manufacturers, particularly in India, from supplying delamanid at lower prices to enable rapid scale-up of this drug.
Paediatric formulations of bedaquiline: dispersible 20mg tablets produced by Johnson & Johnson (J&J) are available through the GDF at a price of $200 for a six-month treatment course for children between five to 12 years old, weighing at least 15kg. For children and adolescents over 12 years old, J&J’s adult 100mg tablets are available through GDF for $270 for a six-month treatment course. Prices of both these bedaquiline formulations remain too high to allow the scale-up of DR-TB care in children, especially for those in need of regimens combining bedaquiline and delamanid.



