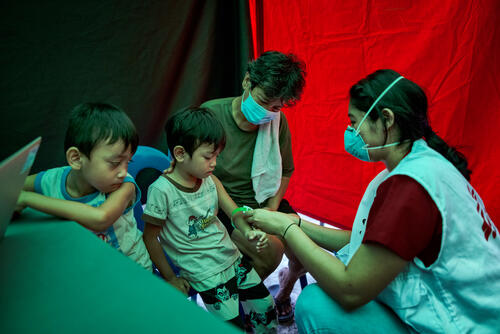
- An Médecins Sans Frontières (MSF) study shows a new all-oral regimen is safer and more effective at treating multidrug-resistant tuberculosis (MDR-TB) than the current options.
- The new findings published in the New England Journal of Medicine today show the new regimen has cured 90 per cent of patients with DR-TB.
- To ensure the new regimen is accessible to everyone, MSF calls on Johnson & Johnson to bring down the price of key drug bedaquiline.
London – A new all-oral, six-month treatment regimen is safer and more effective at treating multidrug-resistant tuberculosis (MDR-TB) than the current options for people with drug-resistant TB (DR-TB), according to the results of an MSF study published in the New England Journal of Medicine today.
These findings are a result of MSF’s TB-PRACTECAL, the first-ever multi-country, randomised, controlled clinical trial to report on the efficacy and safety of a six-month, all-oral treatment regimen, which was recommended in the updated World Health Organization’s (WHO) global TB treatment guidelines released last week. This publication marks the first time TB-PRACTECAL results have been published in a peer-reviewed medical journal.
Until relatively recently, there were no new treatments for TB introduced for over 50 years. Why? Because the disease doesn’t affect people who have the resources to deal with it.Dr Bern-Thomas Nyang’wa, MSF medical director and chief investigator of the trial
“We’re delighted that the trial results have been published in the New England Journal of Medicine after a stringent peer review process,” says Dr Bern-Thomas Nyang’wa, MSF medical director and chief investigator of the trial.
“This publication will provide deeper evidence to policymakers and treatment providers deciding to use the TB-PRACTECAL regimen, in addition to the WHO recommendations,” he says.
“Until relatively recently, there were no new treatments for TB introduced for over 50 years. Why? Because the disease doesn’t affect the people who have the resources to deal with it,” says Dr Nyang’wa. “This trial was an attempt by MSF to try to fill that gap. Now it is essential that the new treatment is made available to everyone who needs it.”
The trial ended enrolment in March 2021, with 552 patients overall and took place in seven sites across Belarus, South Africa, and Uzbekistan. Now, five countries where MSF run activities have begun implementing the shorter regimens with almost 400 patients starting the treatment and eight more countries set to implement it in 2023.
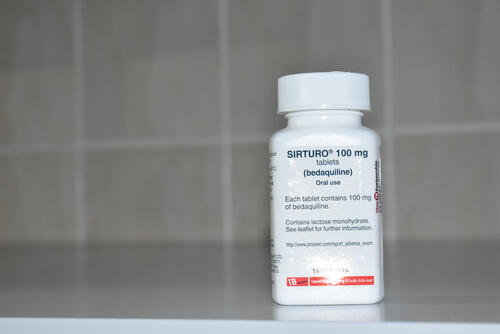
Launched in 2017, TB-PRACTECAL tested three combinations of new treatments against the locally accepted standard of care. All were shown to be favourable against the standard of care. A six-month regimen of bedaquiline, pretomanid, linezolid, and moxifloxacin (BPaLM) proved most effective and safe.
The trial also studied a bedaquiline, pretomanid, and linezolid (BPaL) regimen; and a bedaquiline, pretomanid, linezolid, and clofazimine (BPaLC) regimen.
“We began the TB-PRACTECAL clinical trial nine years ago because something had to be done,” says Dr Nyang’wa. “Patients were telling us that the previous regimens were lengthy and gruelling, and that the side effects were worse than the disease itself.
“They also weren’t very effective; just one in two people were cured. The new regimen, BPaLM, has an 89 per cent cure rate, is safer, shorter, and more tolerable with many fewer pills,” he says.
MSF’s phase II/III clinical trial found that the new shorter BPaLM treatment regimen was very effective against rifampicin-resistant TB (RR-TB), and safer than the current standard of care. Eighty-nine per cent of patients in the BPaLM group were cured, compared to 52 per cent in the standard of care group, and there were fewer recorded side effects in the BPaLM group than the standard of care group. BPaLC and BPaL also performed significantly better than the standard of care.
The new regimen, BPaLM, has an 89 per cent cure rate, is safer, shorter, and more tolerable with many fewer pills.Dr Bern-Thomas Nyang’wa, MSF medical director and chief investigator of the trial
“When I learned that I had TB, I just couldn’t believe it was true,” says Abdirakhman, a 24-year-old mathematics student in Uzbekistan who had to stop studying when he was diagnosed. “[Being diagnosed with DR-TB] was devastating,” he says.
“I chose to join the TB-PRACTECAL clinical trial and was randomly selected to receive the short treatment course of six months compared to the standard two years or more. There were difficult periods, but it was still better than two years of treatment. Now I have finished my course of treatment, and I am back at university,” he says.
While this new regimen provides hope for the 500,000 people who fall sick each year with DR-TB, the current lowest global price provided to the Global Drug Facility (GDF) for a six-month treatment course of BPaLM is around $600, still above the $500 ceiling price called for by MSF.
One of the other newer TB drugs, bedaquiline – developed by Johnson & Johnson with substantial government and philanthropic support – is priced at $270 for six months as the lowest global price. This is despite the fact that researchers have estimated that bedaquiline could be produced and sold at a profit for less than $102 for six months. In fact, all three regimens studied in TB-PRACTECAL are likely to reduce treatment costs compared to the current standard of care.

“Rolling out the shorter, safer, and more effective BPaLM treatment regimen trialed in TB-PRACTECAL could transform the lives of people with TB, but only if the drugs in this regimen are affordable,” says Christophe Perrin, TB advocacy pharmacist with MSF’s Access Campaign.
“As significant public funds helped to pay for the development of bedaquiline, we’re calling on Johnson & Johnson to bring down the price of this drug so that a complete DR-TB treatment course is no more than $500 per person. Too many lives have been lost due to this killer disease. People with TB deserve urgent access to shorter, safer, and affordable treatments,” says Perrin.
MSF is one of the largest non-governmental providers of TB treatment worldwide. In 2021, 17,221 people in our care were started on TB treatment, including 2,309 people with DR-TB. We are working closely with national TB programmes, ministries of health, and other key stakeholders to ensure that this new regimen is available to people with DR-TB as soon as possible.