- Following strong results from an MSF clinical trial into shorter, all-oral treatment regimens for multidrug-resistant TB, WHO is updating its guidelines.
- New guidelines will potentially help millions of patients, but governments must ensure treatment is available.
- To help scale up, MSF urges drug companies producing the regimens’ drugs to drop prices to ensure lifesaving treatment is accessible to all.
Geneva - The World Health Organization (WHO) has announced it will update global guidance on treatment of multidrug-resistant tuberculosis (MDR-TB), following the results shared from the TB PRACTECAL trial. The clinical trial from Médecins Sans Frontières (MSF) has found a new, safer and more effective six-month oral treatment regimen for MDR-TB.
Following the results of our trial, WHO is now recommending programmatic use of the six-month BPaLM regimen – comprising of drugs bedaquiline, pretomanid, linezolid (600 mg) and moxifloxacin – in MDR-TB patients in place of longer, existing regimens. WHO is also recommending another shorter treatment, the BPaL combination (without moxifloxacin), in patients with increased drug-resistance as both regimens showed high treatment success.
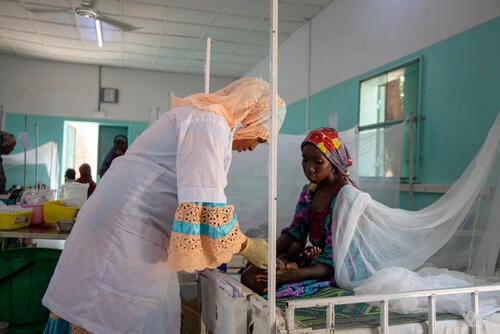
Launched in 2017, TB-PRACTECAL is the first-ever multi-country, randomised, controlled clinical trial to report on the efficacy and safety of a six-month, all-oral regimen for MDR-TB. The trial enrolled 552 patients overall in seven sites across Belarus, South Africa and Uzbekistan. The phase II/III clinical trial found that the new shorter BPaLM treatment regimen was very effective against rifampicin-resistant TB. Results showed 89 per cent of patients in the BPaLM group were cured, compared to 52 per cent in the standard care group.
“When we embarked on this journey nine years ago, patients with MDR-TB around the world were facing lengthy, ineffective and gruelling treatment that disrupted their lives,” says Dr Bern-Thomas Nyang’wa, MSF Medical Director and Chief Investigator of the trial. “So, we were compelled to pursue new treatment options ourselves.”

“These results will give patients, their families and healthcare workers worldwide, hope for the future of MDR-TB treatment,” says Nyang’wa. “We welcome WHO’s decision to update the treatment guidelines; now it is essential that national TB programmes and ministries of health ensure that this treatment is available to people with MDR-TB as soon as possible.”
“[The shorter treatment] would mean a lot as I think when you are on treatment, some parts of your life feel like they are put on hold,” says Awande Ndlovu who was enrolled in the trial at the THINK Hillcrest Clinical Trial Unit in South Africa. “Before [the trial] gave me hope, I couldn’t even see the slightest glimpse of recovering from MDR-TB.”
While this new regimen provides new hope for the 500,000 people who fall sick each year with MDR-TB, the current lowest global price for a 6-month treatment course of BPaLM is US$800. This is still too high and could slow the uptake of the BPaLM regimen in high TB burden countries.
“The groundbreaking TB PRACTECAL results found a more effective and safer regimen for people affected with rifampicin-resistant forms of TB, but we will only see meaningful changes if treatment is affordable,” says Christophe Perrin, TB advocacy pharmacist with MSF’s Access Campaign. “Given the significant public funds that helped to pay for the development of both pretomanid and bedaquiline, these drugs should be affordable and accessible for everyone who needs them.”
Pretomanid – which was developed by TB Alliance and Viatris (previously Mylan) with the help of public funding – is priced at $336 for six months of treatment. Additionally, one of the other newer TB drugs, bedaquiline – made by Johnson & Johnson – is priced at $270 for the same time period. These two drugs together account for three-quarters of the price of the full treatment despite the fact that researchers from the University of Liverpool have estimated that generic versions of pretomanid and bedaquiline could be produced and sold at a profit for less than $210 for six months, and less than $102 for six months, respectively.*
“We call on the TB Alliance, Viatris, and Johnson & Johnson to bring down the price of pretomanid and bedaquiline to ensure that the price of a complete MDR-TB treatment course is no more than $500 per person,” says Perrin. “Price should never be a barrier to accessing lifesaving treatment.”
*Estimated generic prices for novel treatments for drug-resistant tuberculosis, Journal of Antimicrobial Chemotherapy.
MSF and TB
MSF is one of the largest non-governmental providers of TB treatment worldwide. In 2020, MSF started 13,800 people on TB treatment, including 2,100 with drug-resistant TB.
TB-PRACTECAL
TB-PRACTECAL is a multi-arm, multistage, open label, randomised controlled trial which enrolled into three investigational regimens in Stage 1: B-Pa-Lzd-Mfx, B-Pa-Lzd-Cfz and B-Pa-Lzd and a control arm. Stage 2 enrolled into the B-Pa-Lzd-Mfx investigational arm and the standard of care arm only. The trial enrolled 552 patients overall, of which 301 were included in this stage. Current patients in the trial will be followed up until Aug 2022 and the trial will close in Dec 2022. MSF intends to publish data on all 552 patients and arms at that point. More information on TB-PRACTECAL including primary outcome measures can be found here: Pragmatic Clinical Trial for a More Effective Concise and Less Toxic MDR-TB Treatment Regimen(s). MSF and its TB PRACTECAL partners continue to provide care and check-ups for patients who are finishing their treatment in the trial, with the last patient follow-up scheduled for August 2022.