68
68
Belarus is listed as a high-burden country for MDR-TB in the World Health Organization’s 2019 Global Tuberculosis Report.
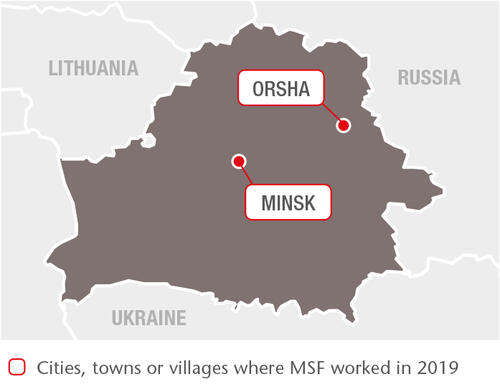
In 2019, we supported the Ministry of Health in four TB facilities in Minsk, the capital, and in Volkovichi village in Minsk region. Our teams also regularly visited a penal colony in Orsha to assist with the treatment of inmates with drug-resistant TB (DR-TB) and co-infections. By the end of 2019, 54 patients with hepatitis C had received treatment with direct-acting antiviral drugs.
MSF, together with the Ministry of Health, devised a harm-reduction programme whose aim is to help patients with DR-TB and alcohol-use problems to manage their dependency on alcohol and other substances in order to finish their treatment successfully. Our psychosocial support team conducted a total of 4,255 consultations in 2019, with around 70-80 patients receiving consultations each month. We also initiated a study to demonstrate the effectiveness and feasibility of this programme.
Medical research
Minsk is one of the five sites of the MSF-sponsored TB PRACTECAL clinical trial, which is looking into short, innovative MDR-TB treatment regimens.Led by MSF UK and conducted in partnership with the London School of Hygiene and Tropical Medicine, other global leaders in medical research, as well as the ministries of health of Belarus, South Africa and Uzbekistan. TB PRACTECAL is an innovative research project that seeks to find injection-free, short, tolerable, and effective treatments for people with DR-TB. By the end of 2019, 51 patients had been recruited for the trial.
Minsk is also one of the 17 sites of the endTB observational study, which is evaluating the safety and efficacy of the newer TB drugs bedaquiline and delamanid. In 2019, the team continued to follow up the 122 patients enrolled in the study.