Like many other humanitarian organisations, MSF is aware that the quality of medicines and other medical products is not always properly guaranteed on the global market (Road map for access to medicines, vaccines and other health products 2019-2023, WHO).The International Office Quality Assurance (IO QA) coordination team has developed three qualification schemes for the internal approval of:
- medicines
- medical devices, in vitro diagnostics, and laboratory items; and
- specialised medical food.
Principles:
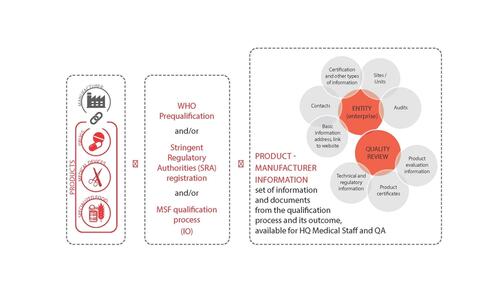
- Qualification is a standardised quality assessment procedure to validate (or not) medical products before they can be purchased for MSF use, by verifying that the product meets at least internationally recognised quality standards. The qualification process consists in the recognition of WHO PQ and/or registration in Stringent Regulatory Authorities (SRA) countries, and when that is not available the assessment of a product dossier and manufacturing site (e.g. GMP audit).
- MSF qualification procedures are based on mutual trust. The manufacturer is asked to certify the veracity and accuracy of the information and documents submitted to MSF. Mistakes or omissions may lead to the disqualification of the product and/or the manufacturer.
- MSF qualification schemes are neither intended to interfere with the World Health Organization’s (WHO) prequalification initiative, nor to duplicate any existing work carried out by SRA countries. Therefore any product that is pre-qualified by WHO (PQ) or that is manufactured and registered and/or used in a Stringent Regulatory Authority country is automatically qualified by MSF.
- MSF is not a Regulatory Agency nor a certifying body. The MSF Qualification Scheme has been designed exclusively for MSF and the decisions taken are only valid for MSF. Decisions are made by using a pre-defined rating system, to ensure impartiality.
Approach:
Products that are neither WHO pre-qualified nor registered in an SRA country and that present an interest for the organisation will be fully evaluated by the MSF QA team. The evaluation is carried out against WHO/FAO norms and standards and/or any other relevant international standard. The evaluation includes the following steps (details are given in the MSF Medical Product Qualification Scheme):
- Preassessment based on product and/or manufacturer questionnaire(s)
- GMP assessment of manufacturing site: The assessment of the manufacturing site for compliance with relevant Good Manufacturing Practices (GMP) is the necessary first step of the evaluation. For medicines, only products that are manufactured in WHO GMP compliant facilities will be evaluated. GMP inspections carried out by the WHO PQ inspectors, or inspectorates recognised by MSF are taken into consideration by MSF. For facilities which have not been previously inspected and approved by WHO PQ or an SRA inspectorate, the MSF International QA Coordinator appoints a GMP expert to perform an audit.
- Product evaluation: for medicines, the assessment is based on product and/or manufacturer questionnaire(s) according to standards set by WHO, and based on a standard Product Questionnaire common to the Interagency Pharmacist Group (UNICEF, ICRC, The Global Fund, WHO procurement center, UNFPA, GDF and MSF).
- Active monitoring and follow up
If the evaluation is successful, a ‘Product Specification Sheet’ (PSS) summarising the characteristics of the product as approved by MSF is issued and is used as a reference for procurement.
MSF qualification is an ongoing procedure and products approved are monitored at regular intervals. Monitoring activities focus on the re-evaluation of the GMP compliance of the manufacturing site, and the updating of the information and data given in the product evaluation.
Expression of interest:
Manufacturers and suppliers of medical products medicines, vaccines, specialised food products, laboratory reagents and equipment, and medical material and equipment are welcome to express their interest to be become an MSF validated supplier, by sending an email to the Assistant to the QA coordinators at [email protected]
Products not included in the MSF list of Medicines, MSF list of Specialised food or MSF list of Medical Devices will not be considered.
Only the manufacturers whose products have been qualified by MSF will be considered for procurement.