Karachi/Paris – Médecins Sans Frontières and Interactive Research and Development (IRD) have announced the start of a clinical trial in Pakistan that aims to find better, shorter treatments against multidrug-resistant forms of tuberculosis (MDR-TB).
The endTB trial is a phase III randomized controlled trial comparing five new treatment regimens against MDR-TB which contain two of the three new tuberculosis (TB) drugs developed in recent years, bedaquiline and delamanid, in combination with other existing oral TB drugs.
A related, parallel trial, called endTB-Q, will evaluate a sixth treatment combination and two different treatment durations, to be used against forms of MDR-TB which are also resistant to fluoroquinolones, a group of antibiotics used as so-called ‘second-line’ drugs in the treatment of TB.
Together, the two trials will identify the most effective and less toxic all-oral, shorter (between six and nine months) treatment regimens against the deadliest forms of TB.
Between its start in March 2017 and the end of September 2019, 344 patients have already been enrolled in the endTB trial across five countries, namely Georgia, Kazakhstan, Lesotho, Peru and South Africa.
Tuberculosis (TB) is the deadliest infectious disease worldwide, with an estimated 1.5 million deaths each year, according to the World Health Organization.
MDR-TB represents a particularly deadly form of the disease. Its treatment requires combining at least four effective drugs for up to 24 months – the length and toxicity of which are often hard to manage. In 2018, only one out of three patients was put under an effective treatment, and among those who were, only half were cured.<a href="https://www.who.int/en/news-room/fact-sheets/detail/tuberculosis> WHO Tuberculosis Fact Sheet 2018</a>
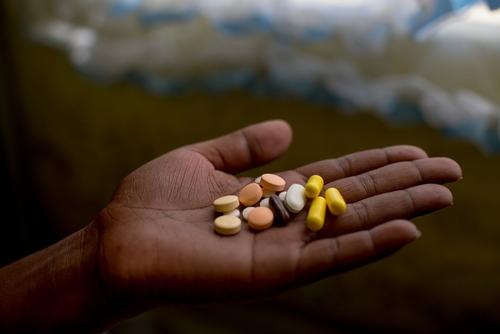
Pakistan is one of the 20 countries with both the highest TB (525,000 estimated cases per year) and MDR-TB burden (27,000 estimated cases per year) globally.
“We are very proud to expand the reach of the endTB clinical trial to this new site in Pakistan, a country with a heavy TB burden”, said Lorenzo Guglielmetti, one of two principal investigators of the trial. “This is another crucial step of this trial, which will build the most robust evidence about the best therapeutic options for the hardest-to-treat TB patients.”
“We remain committed – together with our partner organisations IRD, Partners In Health (PIH) and UNITAID – to offer a better treatment, and a new hope to all MDR-TB patients,” Guglielmetti said.
In Pakistan, the endTB trials will be run at two sites in Sindh province. In Karachi – the first trial site – the first patient was enrolled in the trial in early October at Indus Hospital, while enrolment at the second site is planned to start early next year. The endTB-Q trial is expected to start at both sites in the first quarter of 2020.
In total, 200 patients are expected to be enrolled in the trials at the two sites by the first half of 2021, by a team of over 20 medical staff, working under the joint coordination of IRD and MSF.
The clinical trial in Pakistan leverages on IRD’s experience in working with affected communities and is strongly accompanied by a community engagement component with a three pronged approach: improve TB knowledge in local communities; address stigma, garner support for TB patients and their families; and increase understanding of research amongst local populations, so that they may participate meaningfully.
Expand new drug markets for TB (endTB) is a partnership between Partners In Health, Médecins Sans Frontières, Interactive Research & Development and their funding partner UNITAID. Epicentre, Harvard Medical School and the Institute of Tropical Medicine of Antwerp are partners of the clinical trial.
For more information about the endTB clinical trial: www.endtb.org/clinical-trial
Médecins Sans Frontières (MSF) is an international, independent, medical humanitarian organisation that delivers emergency aid to people affected by armed conflict, epidemics, natural disasters and exclusion from healthcare. Today, MSF has operations in over 70 countries.
MSF has worked in Pakistan since 1986. MSF teams currently run maternal and neonatal care activities in Balochistan and Khyber Pakhtunkhwa; offer care for cutaneous leishmaniasis in four specialised centres in Quetta district and in Peshawar; and provide hepatitis C treatment in Karachi.
IRD is a global health delivery and research organisation based in Singapore that works in 15 countries, including high-burden MDR-TB countries such as Vietnam, South Africa, Pakistan, Indonesia, and Bangladesh. In Pakistan, IRD supports the implementation of innovative strategies by drawing on prior implementation experience in private and public sector facilities and health service delivery initiatives that search, treat and prevent TB. These approaches will contribute in identifying and enrolling patients on both endTB trials.
The Indus Health Network is a network of hospitals and primary care clinics in Pakistan that aims to create an excellence-driven, comprehensive, compassionate, free of charge, replicable healthcare system accessible to all.