While hepatitis C can be cured, few people have access to treatment.
A blood-borne virus that attacks the liver, hepatitis C kills hundreds of thousands of people each year, mostly from resulting complications, usually cirrhosis or liver cancer.
While hepatitis C is found worldwide, the vast majority of people with the disease live in developing countries; China, India, Egypt, and Indonesia are particularly affected.
Hepatitis C is a curable disease, but millions don't have access to treatment due to high prices.

5,810
5,81
Quick facts about hepatitis C


Direct-acting antiviral medicines (DAAs) represent a treatment breakthrough for people with hepatitis C. Yet access to DAAs has been limited because pharmaceutical corporations charge unaffordable prices and, in some places, have blocked the entry of affordable generics with patents. This has led many countries to reserve treatment only for people with the most advanced stages of the disease.


Hepatitis C diagnosis and monitoring is complex, involving multiple tests and resulting in an expensive and resource-intensive process. Laboratory-based tests to measure hepatitis C viral load – needed to confirm active infection and monitor the effectiveness of treatment – are complex, requiring well-resourced laboratories and skilled staff. Simpler, affordable tests that are suitable for use in resource-limited settings are needed to enable access to hepatitis C treatment for all who need it.

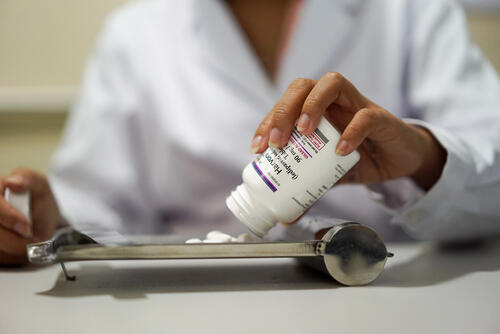
For access to treatment to be scaled up worldwide, the price of DAA drugs urgently needs to come down. Competition among generic producers is already driving down prices, but many countries are not able to access these generic versions because of patent barriers. As a result, countries are having to ration treatment to people. To overcome this and ensure affordable access, MSF is challenging the patent barriers in China and Europe through legal oppositions in court cases.

When Big Pharma plays for keeps, who wins and who loses? (ENG)
When Big Pharma plays for keeps, who wins and who loses?
In this video, we follow the development of sofosbuvir, a true breakthrough drug for hepatitis C, a severe virus that attacks the liver and can be deadly.
The story of sofosbuvir illustrates how research and development for new prescription drugs really works.
After a scientist who got millions in tax-dollars started a small company that did the long work of discovering a new medicine, and showed that it was safe and effective, a giant corporation bought up the next big blockbuster drug and cashed in.
As the new owner of sofosbuvir, Gilead continued clinical trials to learn more about the drug’s effectiveness, safety, side-effects and dosage before seeking government approval to bring the new drug to market.
Gilead first set the price for sofosbuvir at $1000 per pill. That made its worth per gram 67 times greater than gold, but research shows that it only costs $42 to produce.

Persistence reaps rewards in overcoming hepatitis C in Karachi
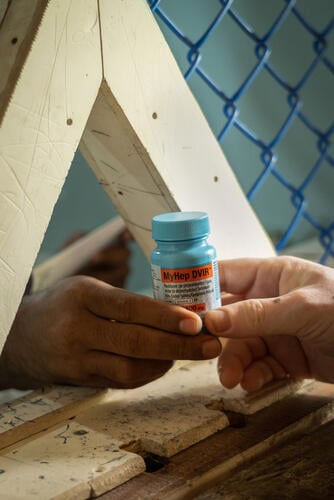
Rohingya in Cox’s Bazar miss out on hepatitis C cure amid lack of treatment options
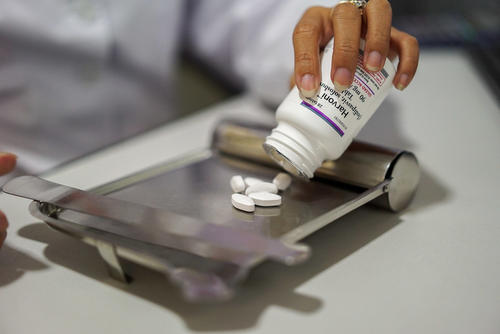
Public health partnership launched to tackle silent epidemic of hepatitis C
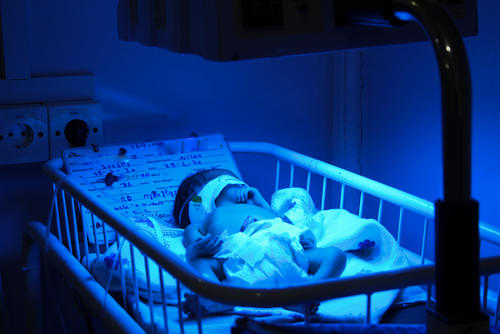
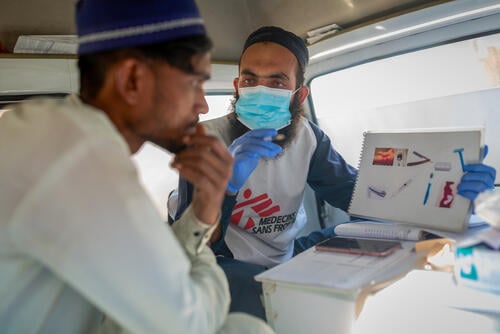
Meeting regular health needs amid the COVID-19 pandemic in Pakistan

Increasing access to hepatitis C treatment in Ukraine

Working to turn the tide of hepatitis C in Karachi’s Machar Colony

Putting people at the centre of TB, HIV and hepatitis treatment in Manipur

Effective drugs and patient support to combat Ukraine’s hepatitis C epidemic

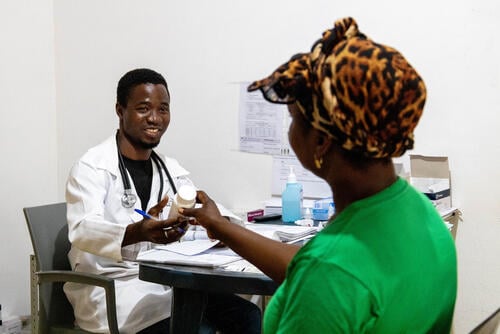
Affordable drugs and new hepatitis C treatment method save lives
Research & Analysis

MSF Field Research
We produce important research based on our field experience. So far, we have published articles in over 100 peer-reviewed journals. These articles have often changed clinical practice and have been used for humanitarian advocacy. All of these articles can be found on our dedicated Field Research website.
Visit site